Composition Analysis of Capsaicin From Habanero Peppers-2004 Report
go.ncsu.edu/readext?443227
en Español / em Português
El inglés es el idioma de control de esta página. En la medida en que haya algún conflicto entre la traducción al inglés y la traducción, el inglés prevalece.
Al hacer clic en el enlace de traducción se activa un servicio de traducción gratuito para convertir la página al español. Al igual que con cualquier traducción por Internet, la conversión no es sensible al contexto y puede que no traduzca el texto en su significado original. NC State Extension no garantiza la exactitud del texto traducido. Por favor, tenga en cuenta que algunas aplicaciones y/o servicios pueden no funcionar como se espera cuando se traducen.
Português
Inglês é o idioma de controle desta página. Na medida que haja algum conflito entre o texto original em Inglês e a tradução, o Inglês prevalece.
Ao clicar no link de tradução, um serviço gratuito de tradução será ativado para converter a página para o Português. Como em qualquer tradução pela internet, a conversão não é sensivel ao contexto e pode não ocorrer a tradução para o significado orginal. O serviço de Extensão da Carolina do Norte (NC State Extension) não garante a exatidão do texto traduzido. Por favor, observe que algumas funções ou serviços podem não funcionar como esperado após a tradução.
English
English is the controlling language of this page. To the extent there is any conflict between the English text and the translation, English controls.
Clicking on the translation link activates a free translation service to convert the page to Spanish. As with any Internet translation, the conversion is not context-sensitive and may not translate the text to its original meaning. NC State Extension does not guarantee the accuracy of the translated text. Please note that some applications and/or services may not function as expected when translated.
Collapse ▲This is a 2004 report from a NC Specialty Crops Program Project. It is posted for historical reference purposes.
PROJECT LEADER(S): Ratna Sharma, Mari Chinn and Michael Boyette
LOCATION: Bioprocess Engineering Laboratory in Weaver Labs at the North Carolina State University Raleigh campus.
IMPACT
The overall goal of the project was to begin preliminary investigations of processing methods for effective capsaicin recovery in effort to redefine habañero peppers as a value added crop for North Carolina farmers. In addition, findings from this research demonstrates the potential of habañero peppers to positively impact the socio-economic development of the state and to provide an alternative valuable crop for North Carolina farmers.
INTRODUCTION
The popularity of chili peppers has been rising over the years, with a large number of growers emerging all over the United States (Coon, 2003). Chilies are grown worldwide, with Asia producing the most per year, followed by Mexico and the U.S. In 2002, 131,950 metric tons of chilies were harvested in the U.S., and New Mexico had the highest domestic production of approximately 91,000 metric tons (NASS, 2003). In comparison to other hot pepper producing states, the production numbers in North Carolina are small and have dwindled with only 650 acres planted in 2001. However, as specialty crops hot peppers have the potential to provide high value products.
Chili peppers, like the habanero (Capsicum Chinese), are a rich source of valuable phytochemicals such as capsaicin. Capsaicin (trans-8-methyl-N-vanillyl-6-nonenamide), an alkaloid or capsaicinoid, is the principal pungent and irritating constituent of hot peppers that are widely used as food additives and possess antimicrobial properties (Jones et al., 1997; Dorantes et al., 2000; Kurita et al., 2002). Capsaicin has been extensively studied via experimental and clinical investigations, due to its prominent pharmaceutical and antioxidant properties (Long and Medeiros, 2001; Rosa et al., 2002). Moreover, capsaicin has been widely used in anti-inflammatory creams and ointments, and as a counter-irritant in surgical dressings and medicines (Kanwar, 2002). It is being formulated into a wide range of topical and nutritional supplements for pain management and arthritic conditions.
Majority of the capsaicin used in the U.S. is imported from other countries. Pure capsaicin rates approximately 16,000,000 Scoville units on the heat index and can sell for over $5000 per gram (Batchelor, 2000). Solid-liquid extraction using solvents like hexane, chloroform, and ethanol is the most commonly employed method for capsaicin recovery (Tapia et al., 1993; Catchpole et al., 2003). However, solvent extraction is energy intensive, leads to problems of toxic waste disposal, and gives a product that requires further purification. Thus, new extraction technologies need to be established for enhancing recovery yields and reducing cost and environmental impact (Yao et al., 1994). Supercritical fluid extraction (SCFE) methods have the potential to address these needed improvements and increase selectivity and purity. This study is being proposed to obtain preliminary data for the investigation of capsaicin extraction from Capsicum Chinese using SCFE. The objectives of the work included: 1) Examining the processing parameters for solvent extraction of capsaicinoids from whole habañero peppers (Capsicum Chinese) and their various parts and 2) Quantifying capsaicin and dihydrocapsaicin levels in whole habanero peppers and their parts using high pressure liquid chromatography (HPLC).
METHODS
The effects of solvent type, pepper parts, tissue preparation, and time on capsaicin and dihydrocapsaicin recovery were evaluated. Habañero pepper samples were dissected and separated into seeds and shells. Moisture for whole peppers, shells and seeds was analyzed using an oven drying method (65°C, 24 hours). The whole and dissected peppers were prepared as fresh, freeze dried, and oven dried for solvent extraction using three solvents (ethanol, acetone and acetonitrile) and conducted in triplicate. Peppers used in the study were obtained from Bailey Farms, Inc. (Oxford, NC) and Cunningham Research Station (Kinston, NC).
Fresh, oven dried and freeze dried preparations (0.5 g dry weight) were extracted using a biomass: solvent loading of 15% (w/v) based on the initial moisture content of the pepper samples/parts. Sample and solvent mixtures were homogenized in 50 ml conical glass tubes and placed in a shaking water bath (50°C). Samples were taken every 20 minutes for a period of one hour. Preliminary studies indicated that capsaicin and dihydrocapsaicin yields were not significantly different at times beyond one hour through 24 hours. Samples were processed by vacuum filtration (Whatman GF/A glass fiber filters, 1.6mm) and stored at -20°C (2 ml aliquots) until HPLC analysis.
The capsaicin and dihydrocapsaicin content of the different samples was quantified using reversed phase HPLC (Discovery® H C18 Column, Supelco, Bellefonte, PA). Capsaicin and dihydrocapsaicin standards were obtained from Sigma and diluted to concentrations of 10, 30, and 50 mg/L to prepare the calibration curve. The samples were eluted at a flow rate of 1 mL/min with a solution of 60% acetonitrile and 40% deionized water adjusted to pH 3 with acetic acid at 30°C. A UV detector was used to quantify the samples at retention times of 7.84 min for capsaicin and 10.20 min for dihydrocapsaicin.
RESULTS
Solvent extractions for the various pepper parts were carried out using fruits from two farms. While the peppers from Bailey Farms were studied for capsaicin and dihydrocapsaicin content in the fresh and oven dried state, the peppers from Cunningham were oven and freeze dried prior to extraction. HPLC analysis indicated that the capsaicinoid yield from seeds was significantly higher than that from shells or whole peppers, irrespective of preparation and solvent. This may be attributed to the fact that capsaicin content is typically high in the placental tissue (Suzuki et al., 1980; Dewitt, 1999). Capsaicin levels of 23.05, 21.82, and 14.30 mg/g of dry tissue were obtained from fresh seeds extracted for 60 min with ethanol, acetonitrile, and acetone, respectively. The capsaicin levels ranged from 14.40 – 16.88 mg/ g of dry tissue for oven dried (Bailey Farms) seeds extracted with the three solvents. Table 1 shows the capsaicinoid levels in extracts of freeze dried seeds and shells prepared from Cunningham peppers.
Table 1. Capsaicin and dihydrocapsaicin levels in solvent extracts collected at 60 minutes from freeze dried seeds prepared from Cunningham peppers
| Solvent | Capsaicin | Dihydrocapsaicin | ||
| (mg/g of dry tissue) | ||||
| Shell | Seed | Shell | Seed | |
| Ethanol | 8.19 | 13.75 | 1.96 | 4.62 |
| Acetonitrile | 7.38 | 16.04 | 2.15 | 5.57 |
| Acetone | 9.73 | 12.14 | 2.12 | 3.83 |
Since dissecting the peppers requires time and manpower, it is proposed that whole peppers be investigated in future studies. Hence, the following presentation of results will focus on extractions from whole peppers.
Bailey Farms
The capsaicin and dihydrocapsaicin levels of fresh whole peppers extracted with ethanol ranged from 7.29 – 9.85 and 2.88 – 4.18 mg product/g of dry tissue, respectively. The results from the extractions are shown in Figures 1 (a) and (b) .These were higher than the 5.26 – 7.74 mg capsaicin/g dry tissue and 1.61 – 2.67 mg dihydrocapsaicin /g dry tissue levels obtained by ethanol extraction of oven dried peppers. The lower levels may be related to the undesirable interactions between the solvent and the water present in the fresh preparations. A similar trend was observed for extractions with acetonitrile. Although, extraction of fresh samples with ethanol for 40 min gave the highest capsaicin levels of 9.85 mg/g of dry tissue, the total amount of capsaicinoids (capsaicin + dihydrocapsaicin) extracted at the end of 60 minutes was highest for oven dried, acetone extracted samples (Fig. 2).
Cunningham Research Station
Freeze dried, whole pepper samples extracted with ethanol and acetonitrile yielded greater capsaicinoid than those in oven dried samples (Figures 3 (a) and (b)). Acetone extractions resulted in capsaicin levels ranging from 9.67 – 10.43 mg/g of dry tissue for freeze dried samples, while the levels ranged from 8.51 – 11.28 mg/g of dry tissue for oven dried samples. As presented in Fig. 4, the maximum yield of capsaicinoids at 60 min was obtained from oven dried samples extracted with acetone and the capsaicin and dihydrocapsaicin levels ranged from 8.51 – 11.28 and 1.71 – 2.75 mg/g of dry tissue, respectively.
Bailey and Cunningham comparison:
A comparison of the solvent extraction efficiencies from oven dried whole peppers, obtained from the two farms, indicates that the capsaicin levels at 60 min were higher in the peppers from the Cunningham Research Station. The dihydrocapsaicin levels were however higher in peppers from Bailey Farms. The slight variation may be attributed to the differences in cultivar, soil type, climate, time of harvesting, and storage period. The total capsaicinoids extracted from oven dried Cunningham peppers ranged from 8.85 – 14.03 mg/g of dry tissue and those from Bailey Farm peppers varied between 9.41 and 11.61 mg/g of dry tissue.
Overall, higher yields were obtained from oven dried peppers using acetone as the solvent. Figure 5 shows the capsaicinoid levels obtained from seeds, shells and whole samples of oven dried peppers subjected to extraction with acetone. As mentioned previously, the capsaicinoid yield from seeds was greater than the shell and whole pepper preparations. This indicates that the concentration of capsaicin and dihydrocapsaicin is greatest in the placental tissue (seeds). It may be expected that the alkaloid contents of the seed and shell samples add up to that of the whole samples. However, the experiment investigated yields from equal dry masses of whole peppers and its parts (shells and seeds). Thus, the composition of the whole pepper (ratio of seed to shell) was not accounted for in the data presented.
TABLES & PHOTOS
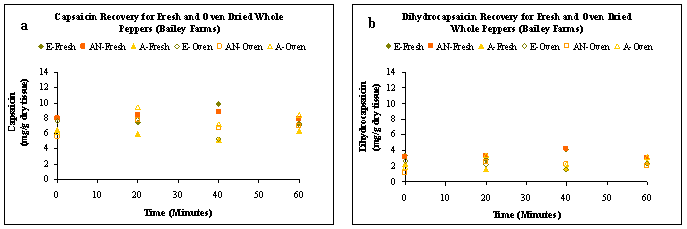
Figure 1: Product recovery (mg/g dry tissue) over extraction time for fresh and oven-dried whole peppers from Bailey Farms a) capsaicin b) dihydrocapsaicin. Ethanol = E, Acetonitrile = AN and Acetone = A.
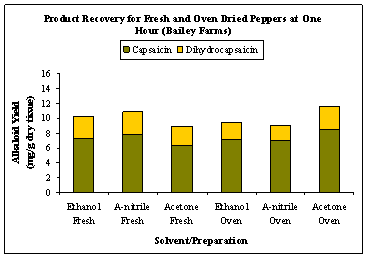
Figure 2: Capsaicin and dihydrocapsaicin recovery for fresh and oven dried whole peppers from Bailey Farms after one hour of extraction using ethanol, acetonitrile and acetone.
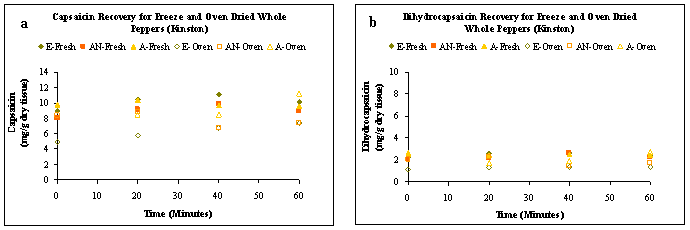
Figure 3: Product recovery (mg/g dry tissue) over extraction time for freeze dried and oven-dried whole peppers from Cunningham Research Station in Kinston a) capsaicin b) dihydrocapsaicin. Ethanol = E, Acetonitrile = AN and Acetone = A..
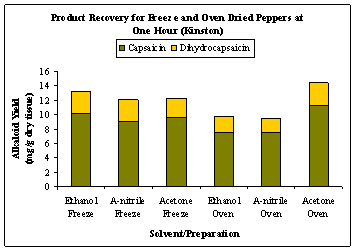
Figure 4: Capsaicin and dihydrocapsaicin recovery for fresh and oven dried whole peppers from Cunningham Research Station in Kinston after one hour of extraction using ethanol, acetonitrile and acetone.
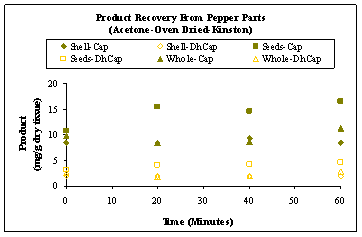
Figure 5: Capsaicin and dihydrocapsaicin recovery over time using acetone from oven dried whole peppers, seeds and shells (Cunningham Research Station, Kinston).
CONCLUSION
The results of the study indicate that habañero peppers contain significant amounts of capsaicin and dihydrocapsaicin and employing an efficient solvent extraction technique can help increase yields.
These results can be used to conduct subsequent research on SCFE as an environmentally friendly, product specific alternative extraction method for capsaicin recovery. In addition, a CO2 gas anti-solvent (GAS) method will be developed to enhance recovery levels.
REFERENCES:
Batchelor, J.D. and Jones, B.T. 2000. Determination of the scoville heat value for hot sauces and chilies: An HPLC experiment. Journal of Chemical Education 77: 266 – 267.
Catchpole, O.J., Grey, J.B., Perry, N.B., Burgess, E.J., Redmond, W.A., and Porter, N.G. 2003. Extraction of chili, black pepper, and ginger with near critical CO2, propane, and dimethyl ether: analysis of the extracts by quantitative nuclear magnetic resonance. Journal of Agricultural and Food Chemistry 51: 4853 – 4860.
Coon, D. 2003. Chile peppers: heating up Hispanic foods. Food Technology 57(1): 39 – 43.
Dewitt, D. 1999. The chili pepper encyclopedia. William Morrow and Company, NY.
Dorantes, L., Colmenero, R., Hernandez, H., Mota, L., and Jaramillo, M.E. 2000. Inhibition of growth of some foodborne pathogenic bacteria by Capsicum annum extracts. International Journal of Food Microbiology 57: 125 – 128.
Jones, N.L., Shabib, S., and Sherman, P.M. 1997. Capsaicin as an inhibitor of the growth of the gastric pathogen Helicobacter pylori. FEMS Microbiology Letters 146: 223 – 227.
Kanwar, K.C. 2000. Eat chilies for healthy reasons. The Tribune 23 August 2000, online ed. Health Tribune section. Available: [http://www.tribuneindia.com/2000/20000823/health.htm#1] Accessed on: 5th December, 2003.
Kurita, S., Kitagawa, E., Kim, C., Momose, Y., and Iwahashi, H. 2002. Studies on the antimicrobial mechanism of capsaicin using yeast DNA microarray. Bioscience, Biotechnology, and Biochemistry 66: 532 – 536.
Long, A.C. and Medeiros, D.M. 2001. Evaluation of capsaicin’s use in analgesic medicine. Journal of Nutraceuticals, Functional and Medical Foods 3: 39 – 46.
National Agricultural Statistics Service (NASS). 2003. Vegetable 2002 Summary. Agricultural Statistics Board, USDA, NASS. Vg 1-2 (03). Available: [http://usda.mannlib.cornell.edu/reports/nassr/fruit/pvg-bban/vgan0103.pdf] Accessed on: 25th November, 2003.
Rosa, A., Deiana, M., Casu, V., Paccagnini, S., Appendino, G., Ballero, M., and Dessi M.A. 2002. Antioxidant activity of capsinoids. Journal of Agricultural and Food Chemistry 50: 7396 – 7401.
Suzuki, T., Fujiwake, H., and Iwai, K. Intracellular localization of capsaicin and its analogues, capsaicinoid, in Capsicum fruit 1: Microscopic investigation of the structure of the placenta of Capsicum annuum var. Annuum cv. Kara Yatsubusa. Plant Cell Physiology 21: 839 – 853.
Tapia, J.C., Garcia, R., Escamilla, E.M., Calva,C., and Rocha, J.A. 1993. Capsaicin recovery from a cell culture broth. Industrial Engineering and Chemical Research 32: 2242 – 2246.
Yao, J., Nair, M.G., Chandra, A. 1994. Supercritical carbon dioxide extraction of Scotch Bonnet (Capsicum annuum) and quantification of capsaicin and dihydrocapsaicin. Journal of Agricultural and Food Chemistry 42: 1303 – 1305.
Reviewed by Jeanine Davis, NC Alternative Crops & Organics Program, Department of Horticultural Science, NC State University on 7/22/2022.








